Umbrella Project I
Cardanol and Glycerol Exploitation
Cardanol is the main constituent of Cashew Nut Shell Liquid (CNSL) that represents one of major source of natural phenolic lipids. Depending on the extraction method, CNSL is classified in two types: CNSL extracted with solvent and technical CNSL. The former has mainly anacardic acid, cardol and, traces of methyl cardol. After the cashew nut being submitted to industrial process of heating, the anacardic acid is decarboxylated rendering technical CNSL which is free of anacardic acid, having as the main constituents cardanol (60-65%), cardol (15-20%) and, polymeric materials (10%). Each phenolic constituent is a mixture of saturated, monoene, diene and, triene compounds. Due to unique characteristics of cardanol and its derivatives, they have been stimulating researchers to study their potential to prepare varnish, coating materials and, as starting material for organic synthesis. The literature has focused to the preparation of hybrid molecular systems where cardanol is involved on the planning of new molecular entities.
Currently, another important industrial residue is glycerol. Its use in the synthesis of higher value chemicals has been highlighted in the green chemistry area for the last few years. The exponential growth of biodiesel production, in which glycerol is generated as by-product raised an enormous concern about its environmental impact. In fact, the glycerol disposal is a major issue in the mass production of biodiesel and it is desirable to find alternatives for the consumption of extra volume of crude glycerol. Thus, glycerol has been used as a versatile primary chemical building block to produce a great variety of commercially valuable compounds.
Taking into account the characteristics of the compounds mentioned above, we aim to combine their properties into single compounds with unique potentials.
Ongoing projects
1. Design and synthesis of fluorescent markers for fuels
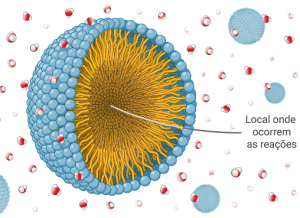
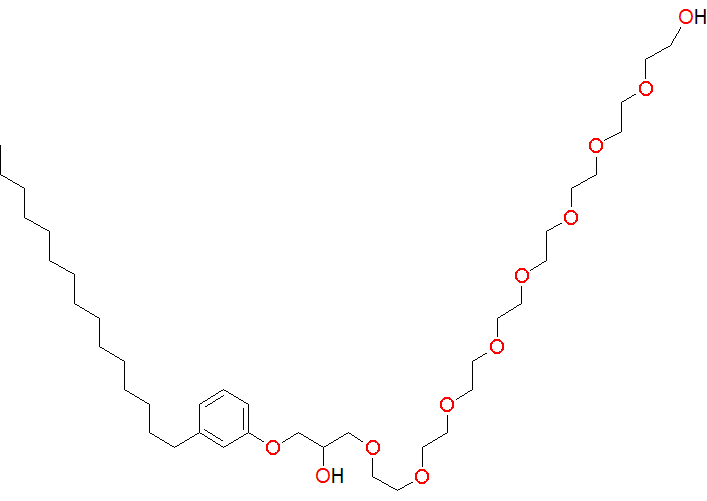
2. Design and synthesis of new surfactants for micellar catalysis – Nanoreactors
This project has been inspired by work of the Lipshutz Research Group, University of California, Santa Barbara.
3. New nonionic amphiphiles to combat the Aedes aegypti mosquito and pathogenic microorganisms
Selected Publications
- Braga, F.; Ojeda, M.; Perdomo, R. T.; de Albuquerque, S.; Rafique, J.; de Lima, D. P.; Beatriz, A. Synthesis of cardanol-based 1,2,3-triazoles as potential green agents against neoplastic cells. Sustainable Chemistry and Pharmacy, 2021, 20, 100408.
- Copini, S. ; MICHELETTI, A. C. ; De Souza, A. M. ; Gomes, R. S. ; De Lima, D. P. ; BEATRIZ, A. Synthesis and Antioxidant and Antimicrobial Properties of β-Hydroxy Sulfides, Sulfoxides, and Sulfones Derived from Cardanol and Glycerol Derivatives. J. Braz. Chem. Soc. 2020, 31, 2569-2582.
- Paiva, D. R.; De Lima, D. P.; Avvari, N. P. ; Arruda, E. J.; Cabrini, I.; Marques, M. R.; Santos, E. A.; Biaggio, F. C.; Sangi, D. P.; Beatriz, A. A potent larvicidal agent against Aedes aegypti mosquito from cardanol. Annals of the Brazilian Academy of Sciences 2017, 89, 373-382.
- Manda, B. R; Avvari, N. P. et al. Synthesis, Antibacterial and Antitubercular Evaluation of Cardanol and Glycerol‑Based β-Amino Alcohol Derivatives. J. Braz. Chem. Soc. 2017.
- Braga, F. C; Avvari, N. P.; Gomes, R. S.; Nascimento, V. A.; Oliveira, S. L.; Caires, A. R.L.; de Lima, D. P.; Beatriz, A. Design, synthesis and fluorescence analysis of potential fluorescent markers based on cardanol and glycerol. Dyes and Pigments 2017, 141, 235-244.
- Beatriz, A. et al. Processo de Síntese de um Composto Iodado e Uso de Outros Derivados do Cardanol como Larvicidas para Controle Populacional do Mosquisto Aedes aegypti e outros Culicídeos. Patent Nr. BR 10 2017 024441 5.
- Schneider, B. U. C.; De Souza, A. M.; Beatriz, A.; Carvalho, P. C.; Mauro, M. O.; Karaziack, C. B.; De Lima, D. P.; Oliveira, R. J. Cardanol: toxicogenetic assessment and its effects when combined with cyclophosphamide. Genetics and Molecular Biology 2016, 39, 2, 279-289.
- Beatriz, A. et al. Processo Eficiente de Purificação do Cardanol Isolado do Líquido da Casca da Castanha de Caju (LCC) e Produção de Derivados de Interesse industrial. Patent Nr. BR 1020140300023